Empirical Formulas — Overview & Examples Expii
Calculate the empirical formula and the molecular formula of this compound given that the molar mass is 188 g/mol. 16. A compound contains 10.13% C and 89.87% Cl (by mass). Determine both the empirical formula and the molecular formula of the compound given that the molar mass is 237 g/mol. 17. A certain compound has an empirical formula of.
Chemistry Empirical And Molecular Formula Worksheet Answers

Show your work for the calculation of empirical formula here Excessive physical activity, lactic acid molecular mass 90.08 g per mole, forms in muscle tissues and is responsible for muscle soreness. Elemental analysis shows that this compound has 40.0% carbon 6.71% hydrogen and 53.3% oxygen. Determine the empirical formula of lactic acid.
Empirical And Molecular Formula Worksheet Answer Key —

Determine the empirical and molecular formula for chrysotile asbestos. Chrysotile has the following percent composition: 28.03% Mg, 21.60% Si, 1.16% H, and 49.21% O. The molar mass for chrysotile is 520.8 g/mol. Answer . Mg 3 Si 2 H 3 O 8 (empirical formula), Mg 6 Si 4 H 6 O 16 (molecular formula)
Empirical Formula and Molecular Formula
Step 1: Calculate the molar mass of the empirical formula (empirical mass) Step 2: Divide the actual formula mass by the empirical mass. Step 3: Multiply the subscripts in the empirical formula by the answer in Step 2. Example: The empirical formula for vitamin C is C3H4O3. Experimental data indicates that the molecular mass of vitamin C is.
Empirical/Molecular Formulas

The empirical formula for this compound is thus CH 2. This may or may not be the compound's molecular formula as well; however, additional information is needed to make that determination (as discussed later in this section). Consider as another example a sample of compound determined to contain 5.31 g Cl and 8.40 g O.
6 Problems Worksheet Empirical and Molecular Formulas

2) Multiply all the subscripts in the empirical formula by the answer to the previous step. Example 2: If the compound from Example 1 had a molecular weight of 306 g, what would the molecular formula be? Solution: The empirical formula was Aℓ 2 O 3. The empirical formula weight is 2 × 27.0 g + 3 × 16.0 g = 102 g
30++ Empirical Formula Worksheet 1 Answers Pdf Worksheets Decoomo

A 100.0g piece was analyzed and found to have 66.5g Cu combined with 33.5g S. To find the empirical formula: 1. Convert from mass to moles using the MM of the element. 2. Repeat for all elements in the compound. 66.5 g Cu x 1 mol Cu = 1.05 mol Cu 33.5 g S x 1 mol S = 1.05 mol S. 63.55 g Cu.
50 Empirical And Molecular Formulas Worksheet
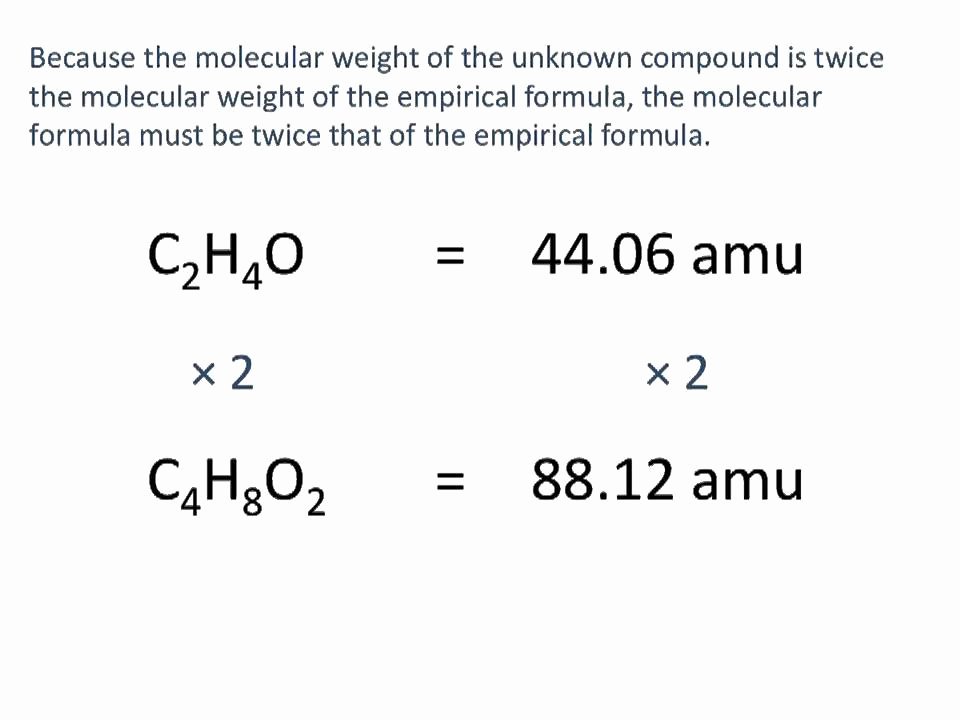
Empirical and Molecular Formulas Worksheet . Objectives: • be able to calculate empirical and molecular formulas . Empirical Formula .. Molecular Formula . 4) Empirical formula of a substance is CH 2O. Molar mass is 180. What is the molecular formula? 5) Sample (3.585g) contains 1.388g of C, 0.345g of H, 1.850g O and its molar mass is 62g..
Free Empirical/molecular Formula Practice Worksheets

19. $3.25. Zip. Use these easy to follow, step-by-step "Placemats" to help students master a tough stoichiometry topic: calculating empirical and molecular formulas. Includes: 12 task cards (featuring differentiated symbols to denote the style and difficulty of each problem), answer key, and cheat sheet handout (1/.
comp empirical and molecular worksheet answer key
Microsoft Word - Answers to Worksheet 8.doc. Worksheet #8. Empirical Formulas. State the empirical formula for each of the following compounds: a) C4H8; b) C2H6O2; c) N2O5; d) Ba3(PO4)2; e) Te4I16. What is the empirical formula for a compound that contains 0.063 mol chlorine and 0.22 mol oxygen?
Free Empirical/molecular Formula Practice Worksheets

56.4%. molecular mass: 284 g/mol. 6. A fat is composed of long chains of carbon and hydrogen atoms. In a. reaction with a strong base, a fat forms a soap and glycerol. What is the. empirical formula of a fat containing 77.60 % carbon, 11.45 % oxygen, and.
Free Empirical/molecular Formula Practice Worksheets

The empirical formula for this compound is thus CH 2. This may or may not be the compound's molecular formula as well; however, additional information is needed to make that determination (as discussed later in this section). Consider as another example a sample of compound determined to contain 5.31 g Cl and 8.40 g O.
Empirical And Molecular Formulas Worksheet
6) A hydrocarbon (a compound that contains only hydrogen and carbon) is found to be 74.5 % carbon by mass. a. Find the empirical formula for the compound. b. If the molar mass of the compound is 16.05 g/mol, find its molecular formula. 7) It was found that a compound contained 68.1% carbon, 13.7% hydrogen, and 18.2% oxygen by mass.
Calculating Empirical Formula Worksheet CALCULATOR GHW

Determine the empirical and molecular formula for chrysotile asbestos. Chrysotile has the following percent composition: 28.03% Mg, 21.60% Si, 1.16% H, and 49.21% O. The molar mass for chrysotile is 520.8 g/mol. Answer . Mg 3 Si 2 H 3 O 8 (empirical formula), Mg 6 Si 4 H 6 O 16 (molecular formula)
Empirical and Molecular Formula Practice by Teach Simple

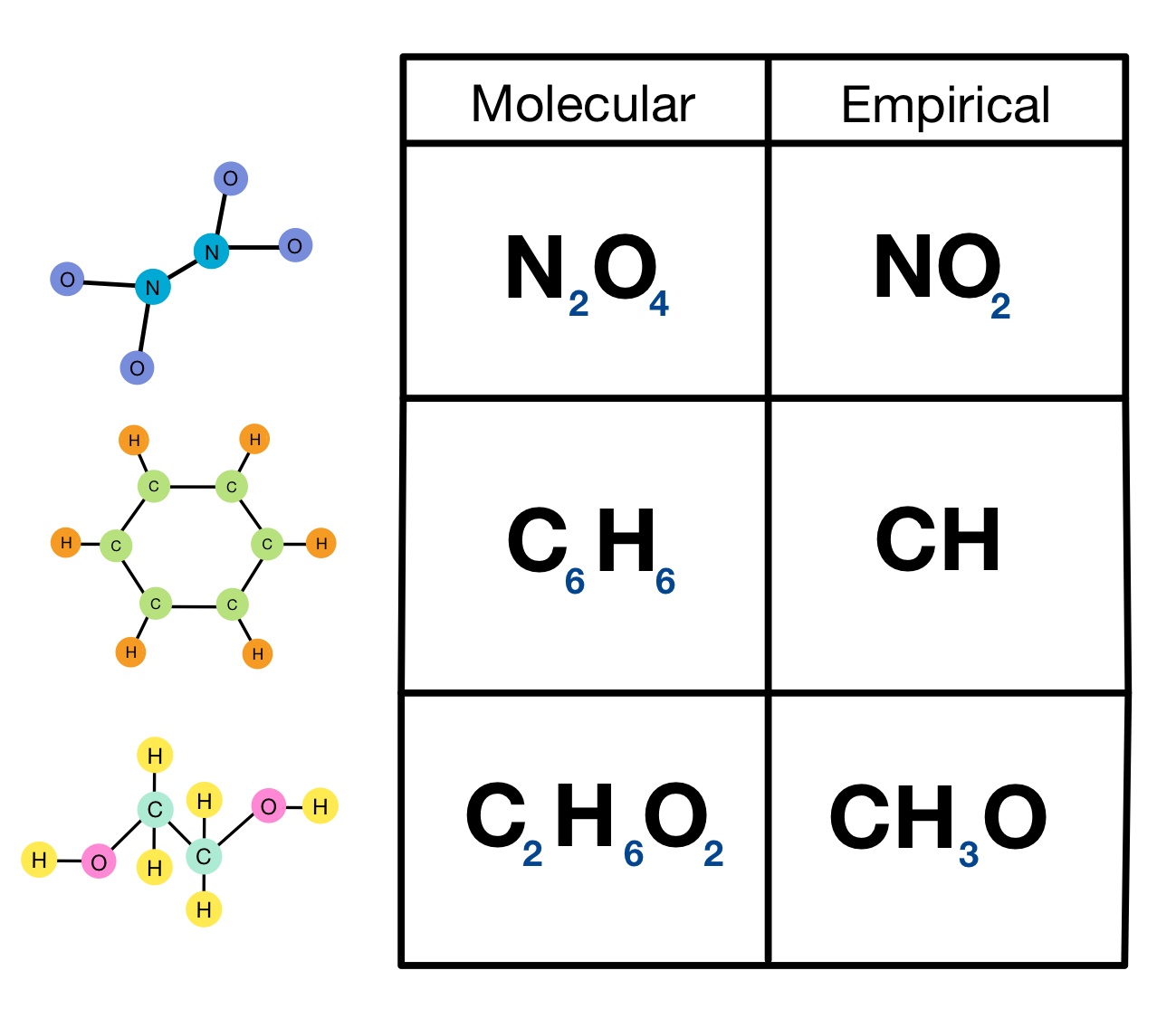
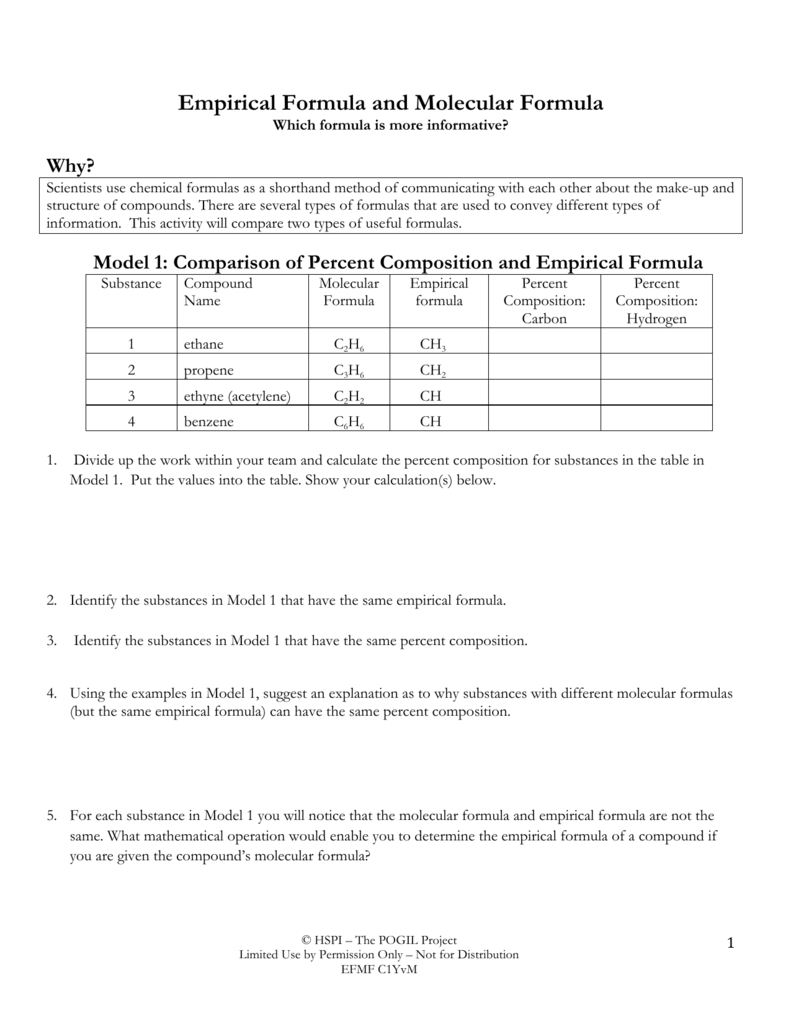
Empirical Formula. The Empirical formula is the lowest whole number ratio of the elements in a compound. In (section 2.10), we discovered that benzene and acetylene have the same mass percent composition, and thus it is logical that they have the same ratio of elements to each other, that is, they have the same empirical formula.. For salts that do not have homonuclear diatomic ions (like Hg 2.
Empirical Formula Molecular Formula Worksheet Worksheets For Kindergarten

To get the empirical formula, just divide each subscript by the greatest common divisor, which in this case is 6, giving the empirical formula as CH. Empirical Formula: CH Molecular Formula: C6H6 12.) A certain sulfur containing amino acid is 33.059% C, 5.551 % H, 11.011 % N, 25.159 % O, and 25.220 % S, by mass.